Author: Xiaolong Xiaojie
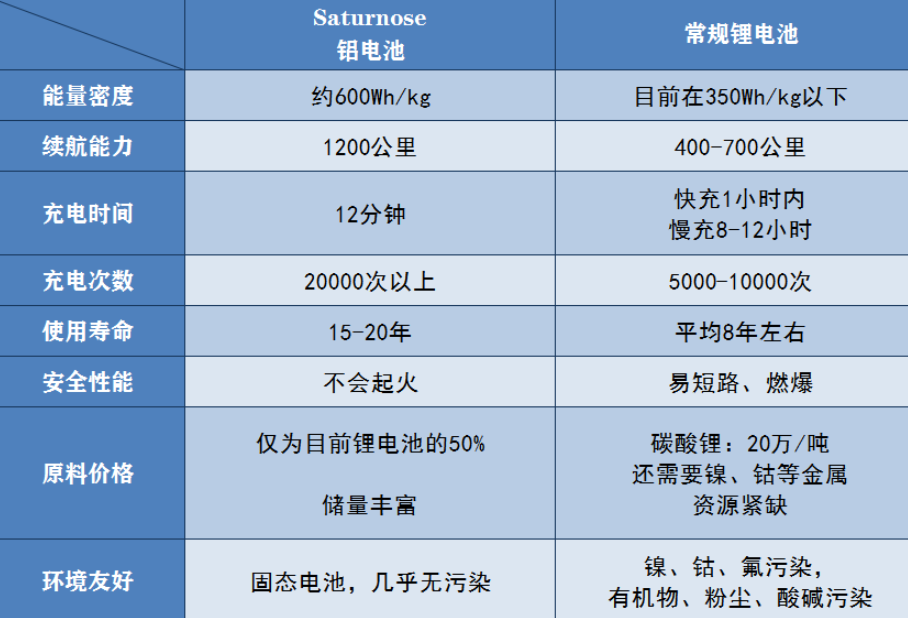
“Will thoroughly change electric cars and energy storage”, Asian battery research and development company Saturnose writes on its website homepage. According to foreign media reports, battery research and development company Saturnose has released an Enhanced Altered Aluminum Ion battery (Ea2I), which has an energy density of over 600Wh/kg, only needs to be charged for 12 minutes, can provide a range of over 1200 kilometers, and can perform over 20000 charge/discharge cycles, which is at least 15 years of stable service life. The company claims that it will achieve the mass production plan of this battery in 2022, becoming the world’s first commercial aluminum-ion solid-state battery.

In today’s mainstream lithium-ion battery field, if the test data of aluminum-ion batteries are true, it will mean a revolutionary advancement in the battery field. When it comes to aluminum-ion batteries, you may feel a bit unfamiliar. What is an aluminum-ion battery, how does it work, and what are its advantages compared to lithium-ion batteries? This article analyzes these three questions.
Understanding Aluminum-Ion Batteries
The lithium-ion battery, which uses lithium cobalt oxide as the positive electrode and graphite as the negative electrode, has always been the “pillar” of energy storage field because of its light weight, high energy density, and long service life. However, against the backdrop of achieving global carbon neutrality goals, the booming development of new energy industry has put forward higher requirements for energy storage devices. Lithium-ion batteries are now facing some challenges due to their limited reserves and expensive prices.
Aluminum metal ranks first in the content of metals in the crust, and its annual mining volume is more than 1,000 times that of lithium metal, with a very low price. In addition, aluminum metal has a high charge storage capacity, and each aluminum atom can release up to 3 electrons in the charging/discharging process, which has a very high energy density. In contrast, lithium can only release one electron. Therefore, aluminum-ion batteries with aluminum as the negative electrode are expected to become the best choice for alternative battery systems.
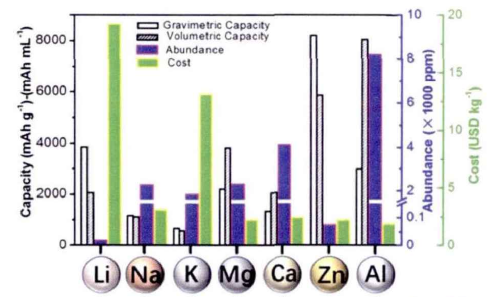
In fact, as early as 1988, the American company Allied-Signal Incorporated launched rechargeable aluminum-ion batteries, but because of technical issues such as the decomposability of its cathode material during charging and discharging, it did not attract much attention at that time. In 2015, a team led by Chinese chemist Dai Hongjie at Stanford University achieved for the first time a rechargeable aluminum-ion liquid battery using aluminum as the negative electrode and graphite as the positive electrode. The paper on the battery was also published in the top international academic journal “Nature”. This battery has several distinct advantages over traditional lithium-ion batteries:
Firstly, the battery uses a three-dimensional graphene as the positive electrode material, which has extremely excellent conductivity and a huge specific surface area. This greatly shortens the charging time of the battery and improves its cycling stability. It is reported that the aluminum-ion battery only takes 1 minute to charge, while it takes 1 hour to charge lithium-ion batteries in phones such as iPhone. Moreover, the aluminum-ion battery has an ultra-long lifespan without any capacity loss after 7500 charge and discharge cycles. This means that if you charge and discharge the battery once a day, the battery will still be as good as new after 20 years.
Secondly, the aluminum-ion battery is also safe. The battery uses ([EMIm]AlxCly) ionic electrolyte, which has low flammability and no safety issues such as easy ignition and explosion. The team members also conducted safety tests on the battery. Even if an electric drill penetrates the battery during charge and discharge, it will not burn and can still function normally for a period of time.
Another prominent feature of aluminum batteries is their flexibility. They can be bent or folded at will, making them potentially useful for flexible electronic devices. Based on these improvements and breakthroughs, “Nature” has also evaluated this research achievement as potentially bringing revolutionary changes to the international battery industry.
The future of aluminum-ion battery is optimistic and worth looking forward to, but there are still some technical problems, mainly:
1) The operating voltage is far from the working voltage of lithium-ion batteries.
2) The storage capacity needs to be improved.
3) The humidity sensitivity of the ionic electrolyte causes its synthesis conditions to be harsh and storage difficulty to increase, and its weakly acidic chemical properties can easily cause corrosion of the battery metal shell.
In addition, the expensive ionic electrolyte inevitably increases the production cost of the battery. Therefore, subsequent research on aluminum-ion batteries mostly focuses on developing new types of positive and negative electrode materials and alternative ionic electrolytes, and has made breakthrough progress. Here, we mainly analyze the most concerned positive electrode materials.
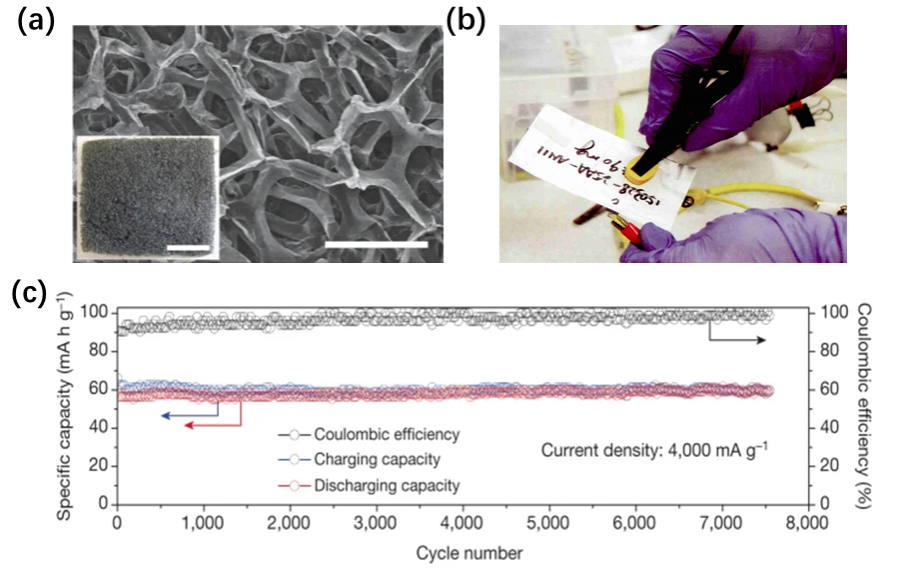
Positive electrode materials in aluminum-ion batteriesBefore discussing the positive electrode materials, let’s briefly introduce the working principle of aluminum-ion batteries.
The working principle of aluminum-ion batteries is similar to that of lithium-ion batteries. Taking graphite as an example, during the discharge process, Al metal is oxidized to form Al3+ at one end of the negative electrode. Under the action of the electric field, Al3+ and [AlaClb]- ion clusters in the electrolyte are embedded into the positive electrode material together to form AlxCly compounds. The AlxCly compound formed by these two ions after embedding also interacts with the graphite positive electrode through van der Waals forces to maintain structural and chemical stability. During the charging process, the embedded AlxCly is extracted from the graphite positive electrode and forms Al3+ and [AlaClb]- ion clusters. Al3+ is reduced to Al metal on the surface of the negative electrode. The shuttle of Al3+ between the positive and negative electrodes, together with the cooperative embedding and extraction of [AlaClb]- ion clusters, constitute the charge-discharge process of aluminum-ion batteries. The specific oxidation-reduction reactions involved are as follows:
Discharge process:
Al – 3e- → Al3+ (negative electrode)
Al3+ + [AlaClb]- + e- → AlxCly (positive electrode)
Charge process:
Al3+ + 3e- → Al (negative electrode)
AlxCly -e- → Al3+ + [AlaClb]- (positive electrode)
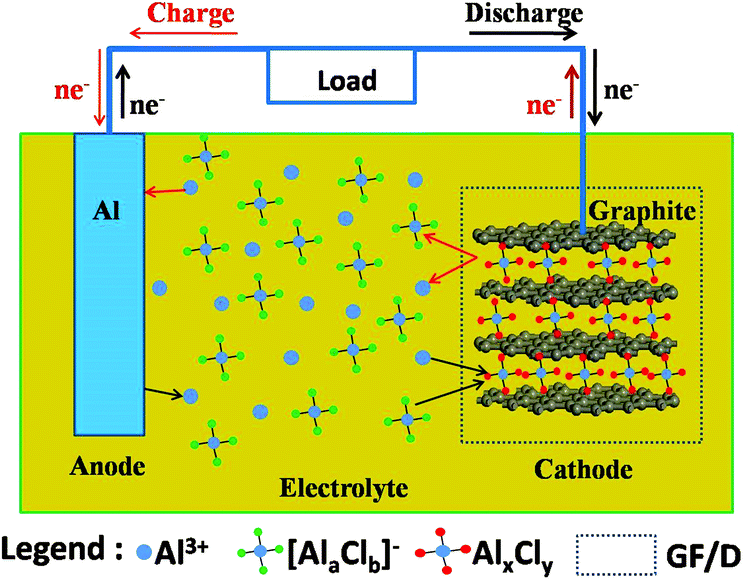
Unlike lithium batteries, Al metal can only release up to three electrons at a time during the oxidation reaction, thus having a higher theoretical energy density. One small detail that needs to be mentioned is that the size of [AlaClb]- ion clusters is much larger than that of Al3+. Therefore, positive electrode materials with a layered structure are often needed for the embedding reaction of aluminum-ion batteries. Overall, the current positive electrode materials for aluminum-ion batteries mainly include carbon-based materials, transition metal oxides, transition metal sulfides, selenides, etc.
Firstly, with regard to carbon-based materials, the three-dimensional graphene positive electrode material adopted by the Dai Hongjie team has achieved ultra-fast charging speed and excellent cycling stability. However, the battery specific capacity and rate performance still need to be improved. To improve specific capacity and rate performance, changes can be made in two directions: altering the structure of carbon-based materials and doping non-metallic elements. The methods of changing the material structure include enhancing the specific surface area, increasing the interlayer spacing, and reducing the intercalation step. Increasing the specific surface area can promote the non-Faraday reaction process, effectively increase the double-layer capacitance, while increasing the interlayer spacing favors the fast insertion and extraction of [AlaClb]- ions, and enhances the rate performance of the battery.Here’s an explanation of the concept of reducing intercalation order. In embedded reactions, the intercalation order represents the content of embedded aluminum ions, usually represented by Stage N, where N represents one embedded layer of ions in every N layers of graphite. For example, when N = 3, it means that one layer of ions is embedded in every 3 layers of cathode material, which means that the smaller the intercalation order is, the more ions are embedded in the embedded reaction, resulting in a higher specific capacity of the battery. Non-metallic doping mainly includes nitrogen and phosphorus doping. Non-metallic doping can change the position of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), thus enabling spontaneous transfer of [AlaClb]- ions from N/P doped carbon to N doped carbon to undoped carbon under built-in electric field formed by HOMO and LUMO, thereby allowing more [AlaClb]- ions to participate in the reaction and increasing the specific capacity of the battery.
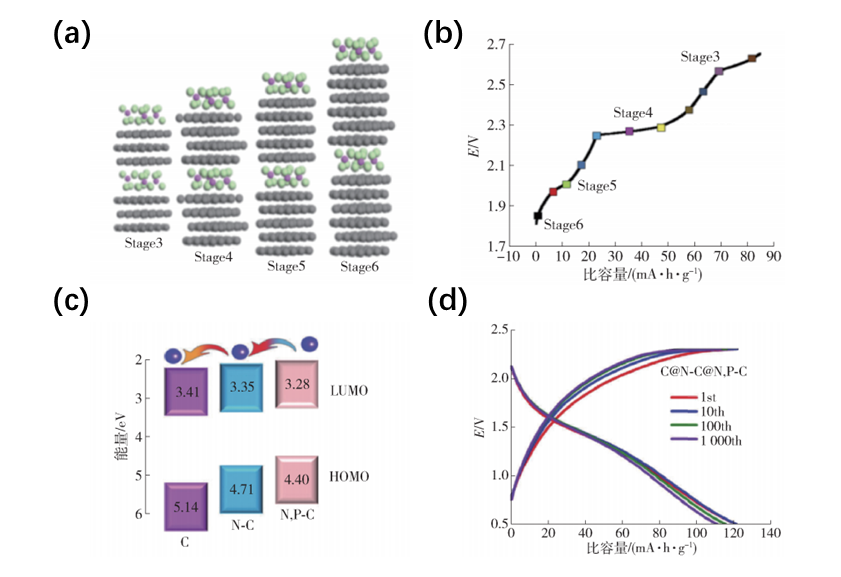
Compared with carbon-based materials, there are fewer reports of transition metal compounds as cathode materials. Although transition metal oxides have a higher initial specific capacity, they generally decay rapidly during cycling due to the strong electrostatic interactions between the oxide and Al3+ ions, which leads to a slow kinetic reaction of aluminum ions and limits the rate performance and cycling stability of the battery to a certain extent. Compared with oxide, the reversibility of Al-S of metal sulfides is better than that of Al-O. However, the decay rate is still very fast, mainly because sulfide materials are easy to dissolve in electrolytes, which seriously affects the cycling stability of the battery. The characteristic of selenide is that the volume of the unit cell is usually larger than that of oxide and sulfide, and the interstitial space between unit cells with the same structure is larger, which is more conducive to the intercalation and deintercalation of [AlaClb]- ions. However, the problem of easy dissolution in electrolytes also exists. In general, the types of cathode materials for aluminum-ion batteries are still very limited, and more effective new compounds are needed to comprehensively improve the electrochemical performance of aluminum batteries.
What is your opinion on Saturnose’s enhanced aluminum-ion solid-state battery?
 At present, Saturnose has not released specific technical details and has only announced some electrochemical parameters. Regardless of its authenticity, “Electric Propulsion” journalists have compared its data with lithium batteries to analyze the advantages it possesses:
At present, Saturnose has not released specific technical details and has only announced some electrochemical parameters. Regardless of its authenticity, “Electric Propulsion” journalists have compared its data with lithium batteries to analyze the advantages it possesses:
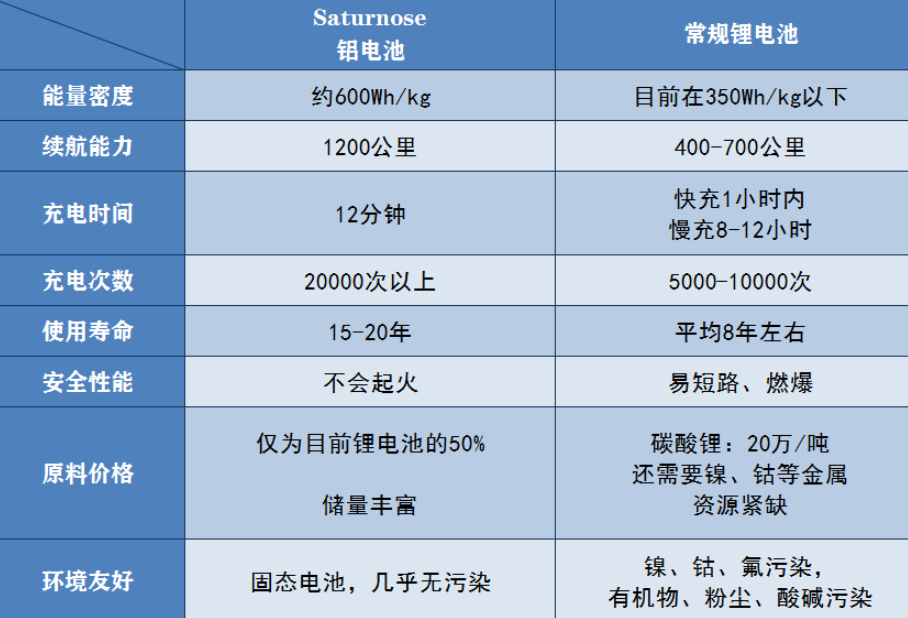
- High energy density. Currently, the mainstream lithium-ion battery in the market is the lithium iron phosphate and ternary lithium-ion batteries. The high nickel material+silicon-carbon negative electrode battery structure can achieve an energy density of about 300 Wh/kg, which is close to the theoretical limit. Even solid-state batteries with higher theoretical energy density, such as NIO’s semi-solid-state lithium-ion battery products released in January 2021, have an energy density of only about 360 Wh/kg. Solid-state lithium-ion battery technology has not yet broken through the technical barrier, and industry experts say it will not be commercialized within 5 years.
On the other hand, Saturnose claims that their enhanced aluminum-ion solid-state battery has an energy density of 550-750 Wh/kg. Even at the lower limit of the energy density of 550 Wh/kg, it is respectively 1.83 times the high nickel ternary lithium-ion battery of Guoxuan High-tech and 1.52 times that of NIO’s semi-solid-state battery, not only breakthroughs the technical barrier of commercializing solid-state batteries, but also far exceeds the energy density of currently mature ternary high-nickel batteries and market-based solid-state batteries.
-
High endurance capacity. High energy density means high endurance capacity. Saturnose claims that “a set of aluminum-ion battery weighing 565 kg and 150 kWh can bring more than 1200 km of endurance range to electric vehicles, with a charging time of only 12 minutes.” In contrast, the full charge endurance of mainstream lithium-ion batteries is generally between 200-400 km. In the case of depleted battery, charging time takes 8-10 hours. Even with fast charging, it requires at least 2 hours. From the data alone, this is undoubtedly a revolutionary progress.
-
High stability and longer cycle life. When providing a 1200 km endurance range, the aluminum-ion battery can perform more than 20,000 charge and discharge cycles, and the electrochemical performance does not decrease significantly, which means its durability is over 15 years. In contrast, the charge and discharge cycle of lithium-ion battery is only 5,000 times, which is only a quarter of that of this aluminum-ion battery.
-
High safety performance. The battery uses a mixture of nano technology to develop fast charging electrodes and electrolytes, and uses aluminum, niobium, and solid electrolytes. The positive electrode material adopts a high-energy, disordered rock salt structure, which avoids problems such as lithium dendrites and thermal runaway fires, and has extremely high safety performance.
-
High cost-effectiveness. Saturnose says, “the (aluminum-ion) battery cost per kilowatt-hour is 50% lower than the current cost of lithium batteries.” Because the battery uses elements such as aluminum and niobium, and does not use nickel and cobalt, and aluminum element reserves are abundant.
Currently, the research on aluminum-ion batteries in academia is still in its early stages, and there is still a lot of room for optimization of various components of the battery, such as the regulation of the oxide film on the aluminum negative electrode surface, the development of new, non-toxic, low-corrosion and inexpensive electrolytes, and the development of positive electrode materials with higher voltage (currently only 2V) and specific capacity. If these issues are fully resolved, along with the advantages and costs of other technical indicators, these low-cost, safe, quick-charge, flexible and long-lasting aluminum-ion batteries will become popular in our daily lives.
It is particularly important to emphasize that due to the characteristics of aluminum-ion batteries themselves, they are unlikely to directly compete with lithium-ion batteries in some application areas that require high energy density. Instead, their low cost, good cycle life and safety make them excel in application areas that emphasize cost, cycle life and safety, such as large-scale smart grid energy storage.
From a commercial perspective, the enhanced aluminum-ion solid-state battery released by Saturnose Company can be said to have come out of nowhere, however, the company has only roughly announced this series of parameters, and the authenticity is doubtful, according to the “Electric Potential” reporter. However, aluminum-ion batteries themselves, as a new type of energy storage device with high specific energy, high cycle life, low cost, high safety, and abundant material reserves, do meet the development trend of batteries today. Regardless of success or failure, the emergence of aluminum-ion batteries provides people with a new possibility and choice. With the deepening of future research on aluminum batteries, it is believed that aluminum-ion batteries will excel in areas that emphasize cost, safety, and cycle life.
This article is a translation by ChatGPT of a Chinese report from 42HOW. If you have any questions about it, please email bd@42how.com.