Introduction: Speak nothing but the truth.
Recently, the patent for “Sodium Metal Battery and Electrochemical Device” of CATL has attracted attention from the media. It is believed that this is a significant change that will subvert the traditional battery industry. According to public patent information, CATL applied for this patent on June 26, 2021, and the announcement date was September 28, 2021. The application number is CN202110742607.7, and the patent is still under review.
The patent application information, which was made public at the end of September, was first reported by financial media such as the Shanghai Securities News, and has since sparked discussion on various platforms. CATL’s stock price, which had been declining steadily since December last year, began to rise steadily on January 11.
One of the reasons everyone is so focused on this patent is that it involves two key points: sodium-ion batteries and sodium-metal batteries. Simply put, a sodium-ion battery replaces lithium ions in traditional lithium-ion batteries with sodium ions. Sodium is abundant and has a much lower price than lithium, making it the most promising next-generation energy storage battery. The sodium metal battery, which everyone is discussing, is a battery without a negative electrode. Although sodium-ion batteries have the advantage of low cost, the energy density that can currently be achieved is only 160 Wh/kg. However, the sodium-metal battery technology can greatly improve its energy density to more than 200 Wh/kg. Industry insiders believe that the combination of these two technologies is a key process that will subvert the traditional lithium battery industry or rewrite history, which means that the era of sodium-ion batteries will arrive even faster in the future.
Let’s analyze these two key points one by one.
The Charm of Sodium-ion Batteries
On July 29, CATL successfully held its first online product launch event. Dr. Zeng Yuqun, Chairman of CATL, unveiled the company’s first generation of sodium-ion batteries and the innovative lithium-sodium hybrid battery pack. As another milestone achievement of CATL’s innovative technology industrialization, sodium-ion batteries will provide a new solution for energy cleanliness and transportation electrification, and promote the goal of “carbon neutrality.”
There were two statements during the launch event that the author found very meaningful – “Energy is the material basis for the development of human society, so the history of energy is also the history of the development of human society.” “Energy conversion and storage are the core of the development of new energy.”Since the Stone Age, people have learned to use natural fire sources, then learned to create fire by friction, and later entered the modern era of fossil-based steam engines during the Second Industrial Revolution, followed by the electric energy era, and now the rapidly developing new energy era. The development of human society is intertwined with the iteration of energy.
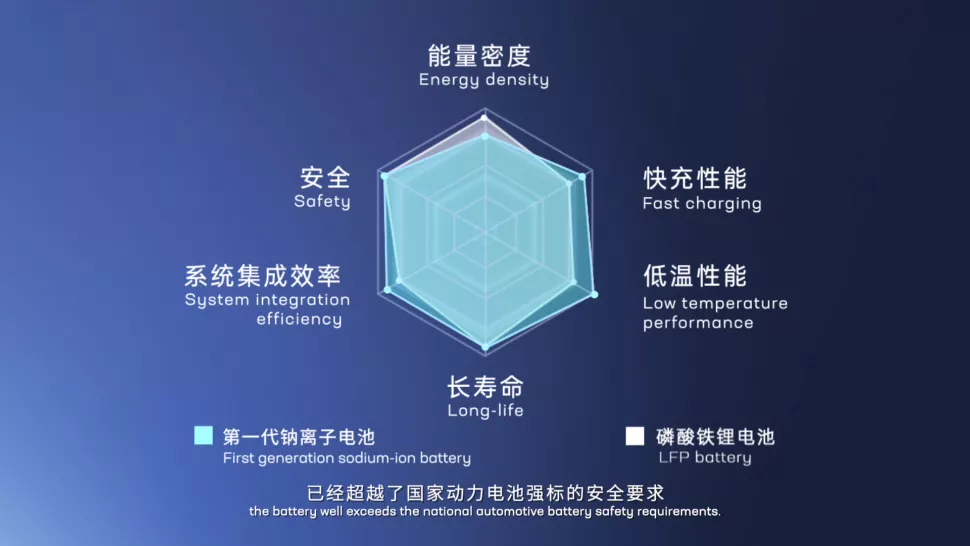
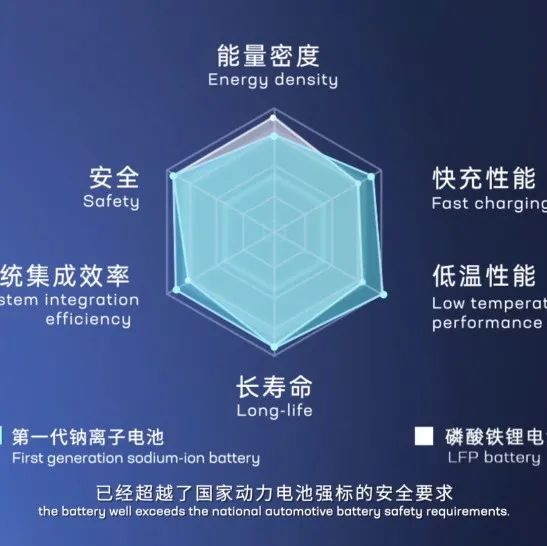
In fact, as early as the 1980s, the research on sodium-ion and lithium-ion batteries started at the same time. However, due to the much lower performance of sodium-ion batteries compared with lithium-ion batteries, especially the energy density, the first generation sodium-ion batteries have an energy density of only 160 Wh/kg (currently the highest in the world), but compared with lithium iron phosphate and ternary batteries, they are still inferior. It’s worth noting that the maximum energy density of ternary high-nickel lithium batteries is expected to reach 350 Wh/kg, while the first generation sodium-ion batteries can only bully lead-acid batteries (energy density about 60 Wh/kg). Therefore, sodium-ion batteries have been ignored for a long time. It was not until around 2010 that a relatively reliable positive electrode material and electrolyte for sodium-ion batteries were developed and the technology received substantial development.
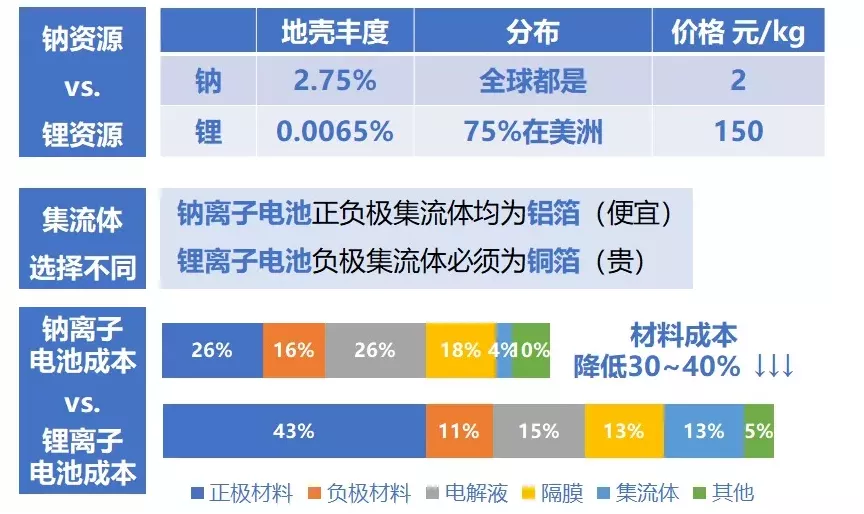
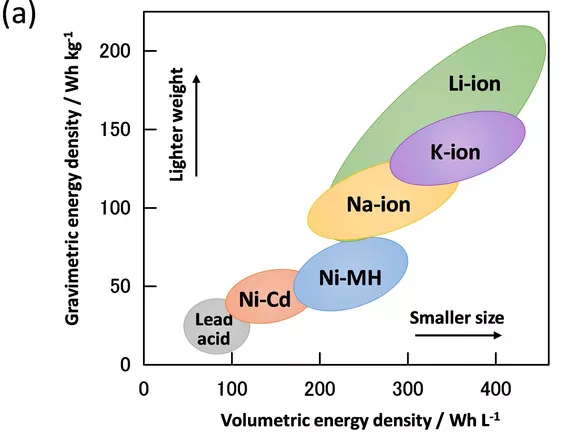
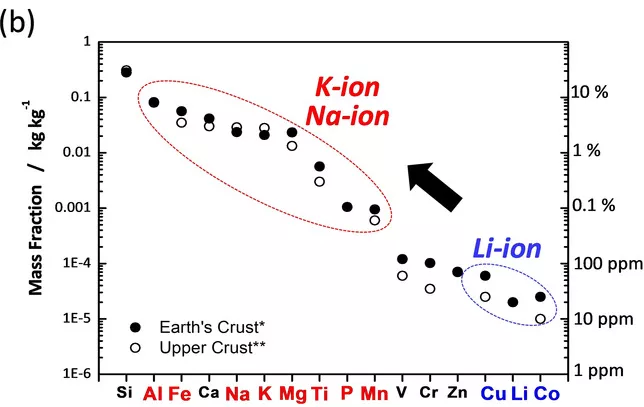
As digital and transportation industries become more dependent on lithium-ion batteries, limited lithium resources will inevitably face a shortage. The research and development of sodium-ion batteries can somewhat alleviate the problem of battery development being limited due to a shortage of lithium resources.
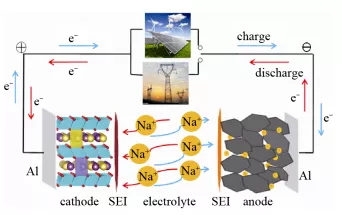 From a theoretical perspective, sodium-ion batteries and lithium-ion batteries are essentially the same, generating electric current through the insertion and extraction of sodium ions from the positive and negative electrode materials during charge and discharge. However, the biggest difference between sodium and lithium ions is that sodium ions have a much larger diameter than lithium ions, which makes it more difficult for sodium ions to pass through the same channel. Furthermore, during multiple insertion and extraction cycles, the collapse of the negative electrode graphite structure is more likely to occur in sodium-ion batteries.
From a theoretical perspective, sodium-ion batteries and lithium-ion batteries are essentially the same, generating electric current through the insertion and extraction of sodium ions from the positive and negative electrode materials during charge and discharge. However, the biggest difference between sodium and lithium ions is that sodium ions have a much larger diameter than lithium ions, which makes it more difficult for sodium ions to pass through the same channel. Furthermore, during multiple insertion and extraction cycles, the collapse of the negative electrode graphite structure is more likely to occur in sodium-ion batteries.
This has raised concerns about the cycling stability of sodium-ion batteries. Lithium-ion batteries can cycle charge and discharge thousands or even tens of thousands of times with little problem (even some with close to 0 capacity loss after several thousand cycles), but sodium-ion batteries typically experience a decrease in capacity of 20% after just a few hundred cycles. Ultimately, this is because sodium ions are relatively large in size and are more likely to cause problems such as electrode material volume expansion, material fragmentation, and sodium ions being trapped during insertion and extraction, leading to irreversible energy loss.
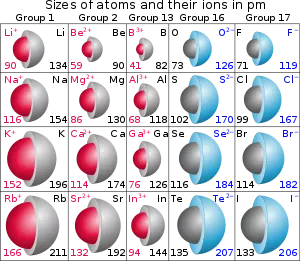
How does a “no negative electrode” battery work?
On December 23, 2014, the Massachusetts Institute of Technology (MIT) in the United States achieved a breakthrough in battery technology. Its newly developed “no negative electrode battery” may overturn the traditional lithium-ion battery industry that has been stagnant for more than 20 years. Its energy density is expected to exceed 1000 Wh/L, equivalent to about 500 Wh/kg. Currently, the biggest bottleneck in the development of sodium-ion batteries is their low energy density, but this technology can make up for this deficiency.
We all know that a battery consists of a positive electrode, a negative electrode, and a separator, but in fact, the positive and negative electrodes are composed of positive and negative electrode materials and current collectors (copper or aluminum foils). A “no negative electrode” battery does not have a negative electrode material (such as graphite), only a current collector serving as a nominal negative electrode. This “no negative electrode” battery technology has been widely publicized along with sodium-ion batteries, but in fact, it was first applied to lithium-ion batteries and can also be promoted in other new types of batteries such as zinc-ion batteries.
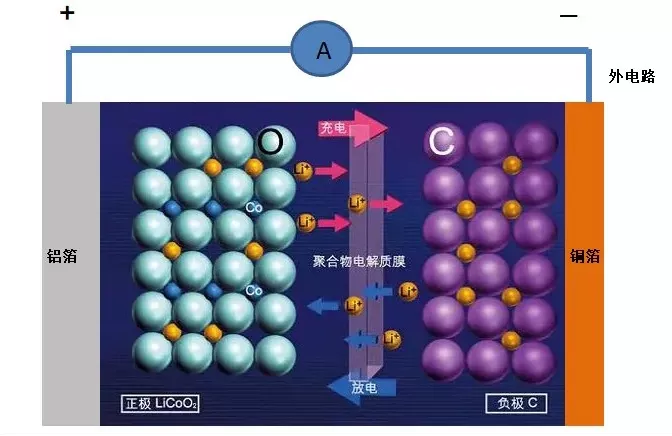
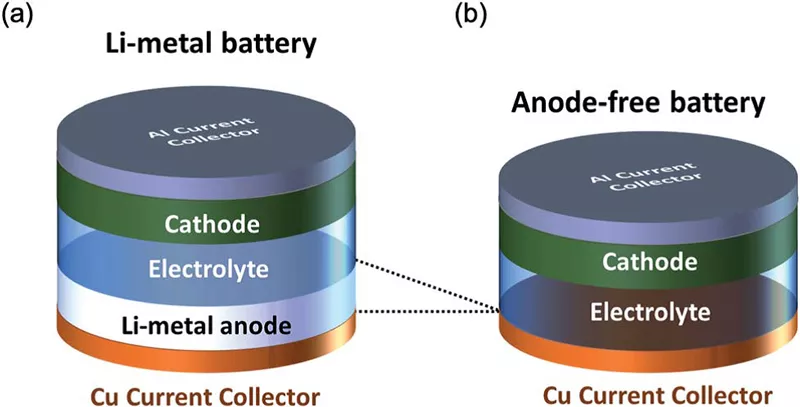 The current collector, as its name suggests, refers to the structure or component that collects the current. In lithium-ion batteries, it mainly refers to metal foils, such as copper foils and aluminum foils. Its function is to collect the current generated by the battery’s active material so as to form a larger current output to the outside. Therefore, the current collector should be in full contact with the active material, and the internal resistance should be as small as possible.
The current collector, as its name suggests, refers to the structure or component that collects the current. In lithium-ion batteries, it mainly refers to metal foils, such as copper foils and aluminum foils. Its function is to collect the current generated by the battery’s active material so as to form a larger current output to the outside. Therefore, the current collector should be in full contact with the active material, and the internal resistance should be as small as possible.
In the so-called negative electrode-free battery, there is no negative electrode material, and the current collector plays a very important role. Because the widely used technology currently is to uniformly deposit a layer of active lithium/sodium on the surface of the copper foil after the first charge and discharge. Uniform deposition is one of the important challenges. The patent technology applied by CATL is on the so-called negative electrode sheet.
Explained by the principle of sodium-ion battery, the departure and insertion of sodium ions are not completely reversible, especially during the first charge and discharge process, and the amount of active sodium remaining on the negative electrode current collector surface is the most. However, in actual cases, the thickness of the deposited active sodium is uncontrollable due to the difference in conductivity or roughness of the surface of the current collector, which is prone to dendrite growth.
CATL starts from the source, making sure that enough residual sodium metal remains after the battery is first charged and discharged to form a layer of uniform and certain thickness of sodium deposition on the current collector’s surface. According to the patent introduction, the thickness of the sodium deposition on the negative electrode after the first charge and discharge of the cell should be greater than or equal to 30 nm, which ensures the stable departure and insertion of sodium ions during the cell’s charge and discharge. CATL’s approach is to pre-set a conductive coating (metal oxide) on the surface of the negative electrode current collector; this can further reduce the overpotential required for sodium deposition, ensuring uniformity of sodium metal deposition after the first charge and discharge. At the same time, this layer of metal oxide protective layer has a nanometer-level thickness, which can form corresponding sodium salts with sodium metal under electrochemical conditions, thereby enhancing the sodium ion transmission rate on the surface of the negative electrode current collector, improving the battery’s dynamic performance, and solving the safety and cycle life problems.Simply put, this production process is like applying a protective film to the negative electrode plate. On the one hand, it is necessary to ensure the thickness and uniformity of the film layer, and on the other hand, to make the film layer have high mechanical strength, so that the structure can maintain its integrity during the volume changes of sodium negative electrode plate caused by charge and discharge and prevent a large amount of sodium dendrites from forming by direct contact with the sodium metal electrolyte.
I searched for patents related to negative-free batteries on CNKI. In fact, such patents have been around for a long time. The reason why the sodium metal patent applied by CATL has attracted so much attention is largely because CATL is a mainstay and leader in China’s new energy industry. Applying for patents is a common practice for companies to protect future possible technologies. It does not necessarily mean that the patented technology will be implemented in large quantities. Many similar patents are only theoretically feasible.
Strictly speaking, the naming of “negative-free battery” itself is problematic and worth pondering, or even feels like playing with words. Why does a battery have to have a positive electrode and a negative electrode? It is because the battery is an energy storage device, which converts electrical energy into electrochemical energy storage during energy storage (charging process), and then converts electrochemical energy into electrical energy (discharge process). As long as you have studied chemistry, you can understand that the energy storage and conversion of the battery are actually a process of redox reaction, and the storage and conversion of energy come from the free energy of the reaction. Therefore, where there is oxidation, there must be reduction; where there is electron loss, there must be electron gain. Now everyone can understand why the concept of “negative-free battery” is not very accurate. This patent of CATL actually uses sodium metal as the negative electrode. This so-called “negative-free” only saves the process of applying and pressing the negative electrode material during the production and assembly of the battery, and instead constructs a more controllable negative electrode on the surface of the negative electrode current collector through the movement of sodium ions during the first charge and discharge process. The negative electrode still exists, and this negative electrode material is the deposited active sodium material.
At the same time, the patent also mentions the uniformity of sodium metal deposition and the avoidance of a large number of sodium dendrites. This is also a technical difficulty, which not only needs to consider the positive electrode material, but also the influence of electrolyte and separator. I think this technology is complex and difficult to control, with great difficulty in mass production. Speaking of safety issues, rather than focusing on this, it is better to pay more attention to the electrolyte. According to the news released at the press conference of CATL’s first generation sodium-ion battery, they also developed special electrolyte materials for sodium-ion batteries. Therefore, I think developing stable electrolyte or solid-state electrolyte is the key.
References1. Kubota K, Dahbi M, Hosaka T, et al. Towards K‐ion and Na‐ion batteries as “Beyond Li-Ion” [J]. The chemical record, 2018, 18 (4): 459-479.
-
Tong Z, Bazri B, Hu S F, et al. Interfacial chemistry in anode-free batteries: challenges and strategies [J]. Journal of Materials Chemistry A, 2021, 9 (12): 7396-7406.
-
Bai S, Sun Y, Yi J, et al. High-power Li-metal anode enabled by metal-organic framework modified electrolyte [J]. Joule, 2018, 2 (10): 2117-2132.
-
Fang Y, Chen Z, Ai X, Yang H, Cao Y. Research progress on positive electrode materials for sodium-ion batteries [J]. Acta Physico-Chimica Sinica, 2017, 33 (1): 211-241.
https://zhuanlan.zhihu.com/p/139775195
- Summary of research results on lithium-free negative electrode batteries
http://www.cailiaoniu.com/216786.html
- Sodium metal batteries, electrochemical devices. QiXinBao
https://www.qixin.com/patents/434e3230323131303734323630372e37/54
- Why are sodium-ion batteries rare?
https://www.zhihu.com/question/26937887
- Announcement of the publication of Chinese patents, State Intellectual Property Office
http://epub.cnipa.gov.cn/Dxb/IndexQuery
This article is a translation by ChatGPT of a Chinese report from 42HOW. If you have any questions about it, please email bd@42how.com.