*This article is reproduced from the autocarweekly WeChat Official Account.
Author: Ao Ao Hu
With the support of the world’s thickest wallets, a group of the world’s smartest minds are fighting against battery life.
Even the most determined electric vehicle enthusiasts cannot claim that the existing battery technology on the market is sufficient for the widespread popularity of pure electric vehicles. Whoever denies this is simply not objective. A true range of 1000 km? Fully charged in 10 minutes? No decrease in performance at low temperatures? Super long cycle life? Half the cost? We want everything, we need everything.
The principle of lithium-ion batteries is not a new concept. The first commercial lithium-ion battery was born as early as 1991. However, advanced automotive power batteries still have room for development, and practical pure electric vehicles have only appeared seven or eight years ago. Therefore, in recent years, with the influx of capital, the battery industry has come up with new concepts one after another.
The ultimate fantasy: solid-state batteries
When it comes to future batteries, solid-state technology is definitely a topic that cannot be avoided. In recent years, as the sales of electric vehicles have surged, we hope that solid-state batteries will make them even better; when the range of electric vehicles is embarrassing, we also hope that the advent of solid-state batteries will solve this issue. In addition, Toyota’s strategy of not trusting existing lithium-ion batteries and betting on solid-state technology has generated a lot of discussion and made solid-state batteries the buzzword.
What does “solid” mean in solid-state batteries? If current batteries are not solid-state, then what are they? Why will solid-state technologies make batteries better, and why haven’t we used them yet?

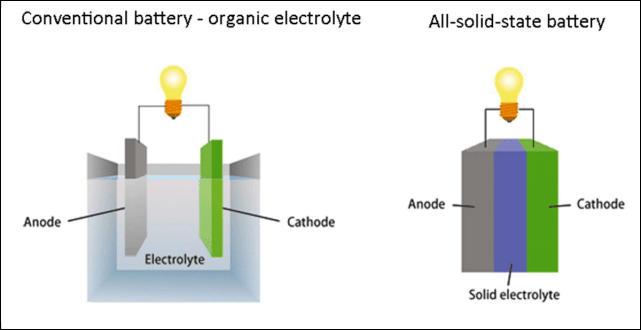
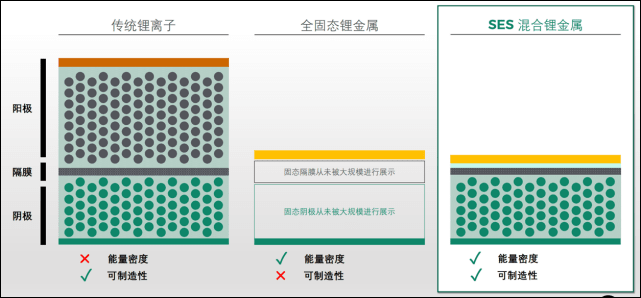
Solid-state batteries are characterized by their solid-state electrolytes instead of liquid ones. All lithium-ion batteries currently on the market for automotive use, although they look like solid metal boxes from the outside, all have liquid electrolytes inside — they are just “fixed” in place by various structural designs so that they won’t easily move around like mineral water.
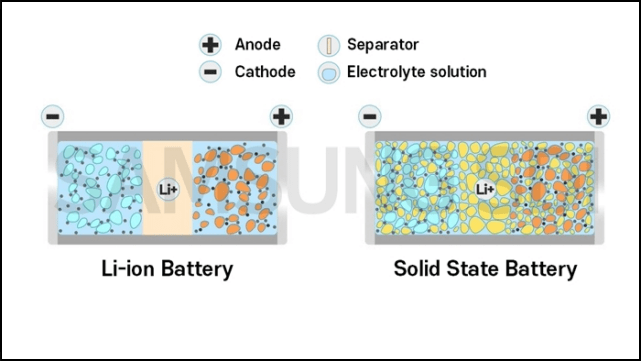
If you still remember your middle school physics, you should at least remember that a battery consists of a cathode, an anode, and an electrolyte.Outside the battery, electrons lost from the negative electrode flow towards the positive electrode via the conductor. Since electrons carry negative charge, the current flows from the positive to the negative electrode. Inside the battery, positively charged ions that lost electrons at the negative electrode flow towards the positive electrode through the electrolyte, while negatively charged ions that gained electrons at the positive electrode flow towards the negative electrode. When we use electricity, only the outside part of the battery is utilized, where the current flows through the conductor and electrical appliance, producing light, heat or power.
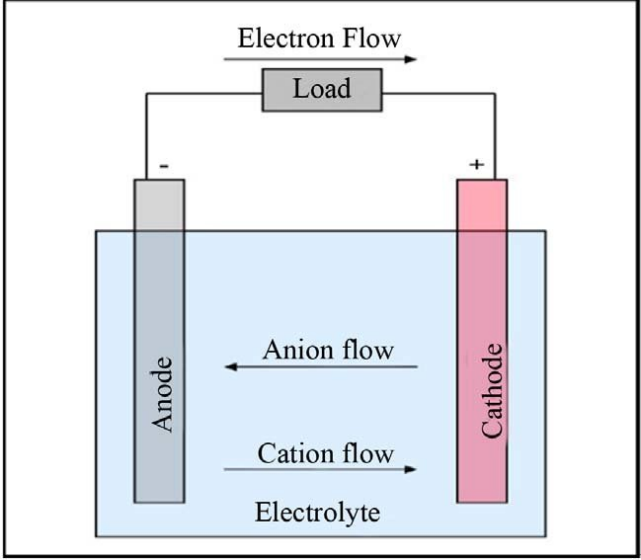
In short, it is a scene where a large number of electrons and ions are constantly migrating. Outside, an electron is released, while inside, a Lithium ion departs. If the flow of ions inside is not smooth, the current outside will also be obstructed, which leads to poor charge-discharge performance. Obviously, liquid is the most convenient medium for ions to move around in, and even if you don’t understand chemistry, you should have seen chemical teachers demonstrating various solutions.
It’s also obvious that changing the electrolyte to a solid state will make it more difficult for ions to flow through. In the past, ions moved from one liquid to another, but now they need to go through a solid material. This is the biggest challenge faced by solid-state batteries at present, as their ability to tolerate large currents for charging and discharging is inadequate. However, high-power discharging and charging capabilities are essential for future electric cars.
However, the temptation of solid-state electrolytes is also noteworthy. Traditional lithium-ion batteries have electrolytes that occupy a considerable amount of weight, whereas solid-state electrolytes can be thinner and lighter, thus increasing the energy density of batteries. Lithium-containing electrolytes are already easy to ignite, and the risk increases if they leak. Therefore, separating the positive and negative poles internally and protecting them externally increases the weight and volume. With liquid electrolytes, it is difficult to use Lithium-metal electrodes to increase energy density.
In other words, transitioning the electrolyte from liquid to solid will significantly improve the energy density and safety of batteries. However, at present, there are still shortcomings concerning fast charging and discharging, cycling life, and preparation costs, which have not been fully resolved for solid-state batteries. Early products may enter the market within the next few years. However, mature solid-state batteries that can be mass-produced may not emerge until 2025 or even later.

NIO ET7’s so-called solid-state battery is actually advertised as “in-situ cured solid-liquid mixed electrolyte”. In-situ curing means that the electrolyte is not initially solid, but after being coated on the electrode, it forms a solid material in situ through certain measures or reactions (specifically unknown). Since there is still some liquid remaining after the process, it is called a solid-liquid electrolyte.The energy density of this semi-solid-state battery, as disclosed by NIO, is 360Wh/kg (single cell/ battery core energy density). This is certainly higher than the current conventional non-solid-state batteries (the current highest is about 300Wh/kg), but not as out-of-reach as our expectations for solid-state batteries, especially considering the more advanced technology used in its positive and negative electrode materials.
Is it fully solid-state? Not quite, but partially. The good thing is that users can indeed enjoy the currently leading energy density of batteries as soon as possible. The downside is primarily on the technical side of the industry, as it cannot fully demonstrate that NIO has sufficient future solid-state battery technology. Of course, the second point is not very sufficient and should not overshadow the first point.
Reshaping the Past: Lithium Metal Anode
One of the advantages of solid-state batteries is that they are more likely to use lithium metal electrodes. Last month, the start-up company SES, which has been studying lithium metal batteries, released a lithium metal battery named Apollo at its first SES Battery World event, with a single-cell energy density of an amazing 417 Wh/kg, and this is only the first-generation product of lithium metal batteries.

The so-called lithium metal batteries refer to the negative electrode materials made of lithium metal. The commonly used negative electrode materials are graphite, and the mainstream development trend is to add silicon, namely silicon-carbon anode (which is also used in NIO’s solid-state battery). The negative electrode material provides electrons to the outside (discharge) and releases lithium ions into the inner electrolyte, so the amount of lithium ions that the negative electrode can “store” will determine the upper limit of the battery energy density.
The “storage” capacity of silicon is more than ten times higher than that of carbon, making it a better choice. However, directly using a silicon anode is not feasible because the volume change of the silicon anode during charge and discharge is too large, and the expansion rate can reach 300%, while graphite is only 10%. Therefore, the current trend is to add as much silicon as possible to the graphite anode, which is called “silicon doping to supplement lithium” in the IMn L7.
Since the negative electrode’s function is to provide lithium ions, why not use lithium metal, which has “built-in” lithium ions, directly instead of looking for carbon and silicon to “store” lithium ions? Compared with carbon and silicon, the lithium metal anode only requires a thin layer, which greatly reduces the weight and volume of the negative electrode. This is an important source of high energy density for lithium metal batteries.
 Actually, lithium metal batteries in a broad sense have been around for a while, but they were originally not rechargeable and can only be used once. This is because although lithium metal is a good negative electrode, when lithium ions need to return to the negative electrode during charging, they will precipitate lithium metal on the surface of the negative electrode in traditional liquid electrolytes. Over time, this precipitation will grow into so-called dendrites.
Actually, lithium metal batteries in a broad sense have been around for a while, but they were originally not rechargeable and can only be used once. This is because although lithium metal is a good negative electrode, when lithium ions need to return to the negative electrode during charging, they will precipitate lithium metal on the surface of the negative electrode in traditional liquid electrolytes. Over time, this precipitation will grow into so-called dendrites.
Over time, the dendrites will grow to a certain extent and may puncture the separator between the positive and negative electrodes, cause dangerous electrolyte leakage from the battery case, or even directly contact the positive electrode material and cause a short circuit. Therefore, in the past, if lithium metal was used as the negative electrode, it was a disposable or short-lived battery that had to be thrown away after use regardless of the dendrite problem.
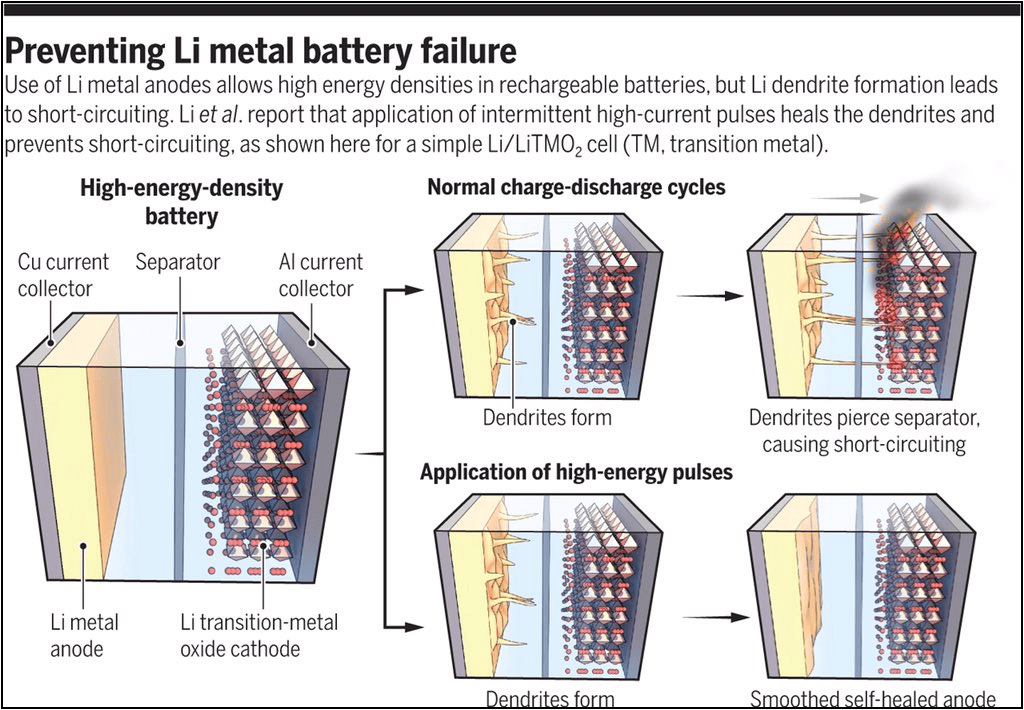
Today’s lithium metal batteries, which can be used in automotive power batteries, are due to the rise and maturity of solid-state electrolyte technology. If the electrolyte is solid, then it can stop sharp dendrites from growing, as the precipitation of lithium in the electrolyte becomes a nuisance. SES’ lithium metal battery adopts a partially solid-state mixed electrolyte, and one of the advantages of solid-state batteries is that they are easier to use with lithium metal negative electrodes. The two can be said to promote each other or even progress together, with the solid-state electrolyte making the lithium metal negative electrode available, and the lithium metal negative electrode making the solid-state electrolyte more advantageous.
Lithium metal negative electrode technology is also still in the early stages, and the extent to which electrolyte solidification solves the dendrite problem still needs time and experience to verify. The estimated time of large-scale production is expected to be after 2025. Automakers who are optimistic about lithium metal batteries are mainly General Motors and Hyundai, who have participated in several rounds of financing for SES.
Ongoing Evolution: High Nickel and Cobalt-Free
Relative to the electrolyte and negative electrode, the positive electrode’s “advanced gameplay” is somewhat monotonous. For ternary lithium positive electrode materials, the main theme has been to increase the nickel content, reduce, and even eliminate cobalt. This trend has continued from NCM (nickel: cobalt: manganese) 523 to 622 and then to 811: nickel proportion has increased to 80%, while cobalt content has been reduced again and again.
Nickel is the direct factor that improves energy density, and its content determines the reversible lithium insertion capacity of the positive electrode. However, of course, the proportion of nickel is not simply increased by increasing it, as too much nickel will cause cation mixing in the positive electrode, with nickel ions and lithium ions occupying each other’s positions, reducing the battery’s cycling performance and lifespan. High nickel will exacerbate the battery’s self-heating phenomenon at high temperatures and cause the internal temperature and pressure of the battery to rise more easily, affecting its safety.
Cobalt, on the other hand, helps batteries improve their cycling life. When lithium ions are reversibly inserted and removed from the positive electrode material during charging and discharging, cobalt can help the layered molecular structure in the positive electrode remain stable. But at the same time, the higher the cobalt content, the lower the reversible lithium insertion capacity of the positive electrode, resulting in a decrease in energy density.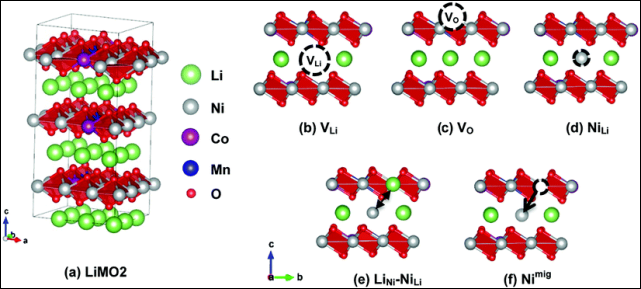
Due to the toxic nature of cobalt, its mining process has been protested by environmental and human rights organizations around the world because of the inhumane practices associated with it. At the same time, cobalt is also very expensive due to the difficulty of mining. Therefore, battery manufacturers have sufficient motivation to reduce the amount of cobalt used in terms of morality and cost.
After years of technological iteration on its part, Tesla has reduced the cobalt content in its ternary lithium batteries to 3%, and will further reduce it to 1% and eventually achieve cobalt-free. To achieve low or no cobalt, other methods must be found to replace the stable effect of cobalt elements in the layered structure of positive electrode materials.
In China, Honeycomb Energy, a subsidiary of Great Wall Motors, became the first company to achieve mass production of cobalt-free batteries this year. Its NMx battery has removed cobalt elements and its single-cell energy density still maintains a high level of 240 Wh/kg. A common method of cobalt-free is doping cations, nanonetwork encapsulation and other methods to improve the layered structure of molecules, replacing the stable role of cobalt elements. The well-known NIO semi-solid state battery also mentions the use of a nano-grade encapsulated super-high nickel positive electrode (low cobalt).

Positive electrode, negative electrode, electrolyte, and even the entire package technology are key factors in improving the overall performance of vehicle batteries beyond just battery cells.
For example, what Tesla is doing – optimizing at the package level by replacing the 2170 battery cell with the larger 4680 specifications and using CTC to reduce structural redundancy while simultaneously achieving non-module CTP, demonstrates that there is still ample room for optimization at the package level even without seeking radical advances in cell technology. However, package-level optimization also depends on progress at the cell level, as without sufficient confidence in its stability, there is no way to talk about CTP and CTC.
If you have sufficient confidence in the speed of battery technology progress, you may start dreaming of a pure electric vehicle with solid electrolytes, lithium metal anodes, high-nickel cathodes, and CTC battery packs.
This article is a translation by ChatGPT of a Chinese report from 42HOW. If you have any questions about it, please email bd@42how.com.
